Step 1 The aldehydes and ketones are the two types of organic functional groups. View the full answer Step 2 Final answer Previous question Next question Transcribed image text: B. Classify the following line-bond formulas as ketones or aldehydes Drag each item to the appropriate bin. Aldehyde Ketone mViewtassignmentPio
Aldehydes, Ketones and Carboxylic Acids – Practically Study Material
Jul 26, 2022search Search build_circle Toolbar fact_check Homework cancel Exit Reader Mode school Campus Bookshelves menu_book Bookshelves perm_media Learning Objects login Login how_to_reg Request Instructor Account hub Instructor Commons Search Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF) Resources expand_more
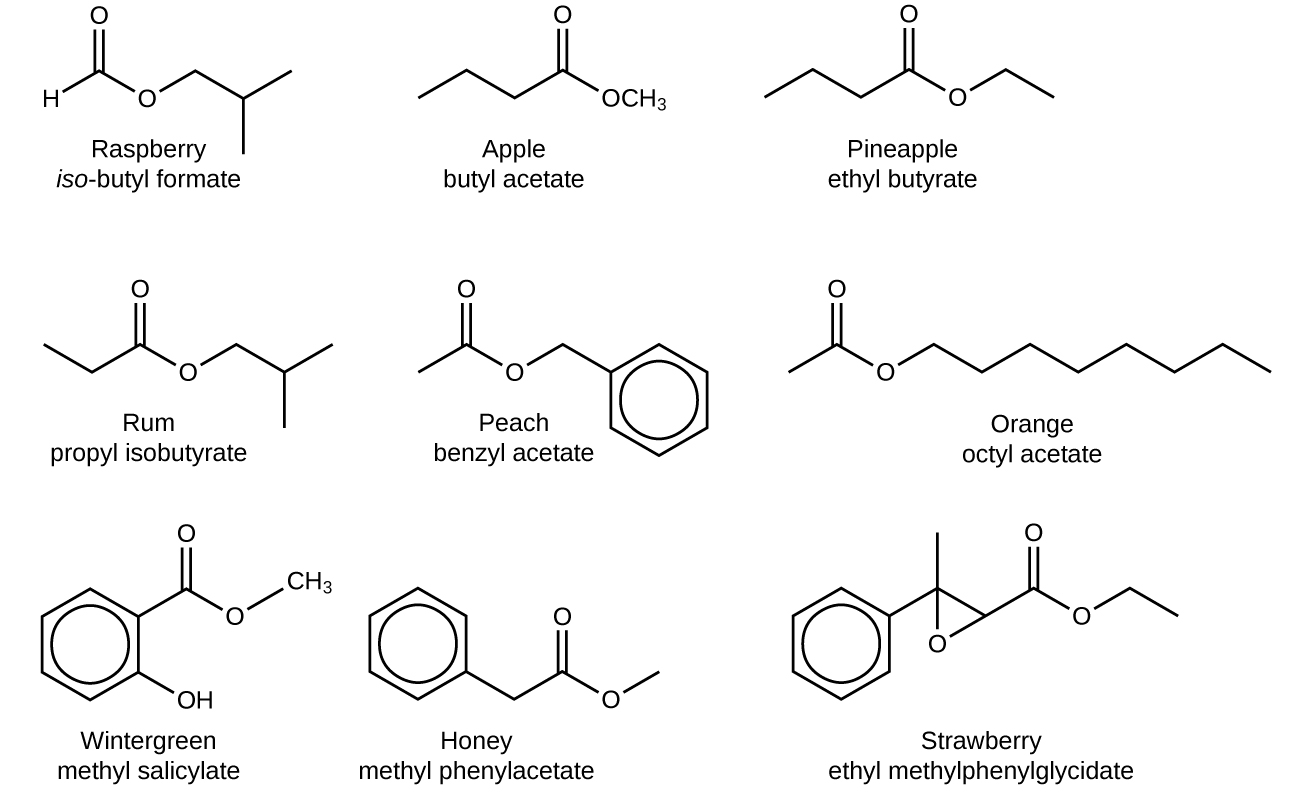
Source Image: ecampusontario.pressbooks.pub
Download Image
1: Aldehydes and Ketones 1.2: Aldehydes and Ketones– Structure and Names Expand/collapse global location 1.2: Aldehydes and Ketones– Structure and Names Page ID

Source Image: slideshare.net
Download Image
SOLVED: Classify the following line-bond formulas as aldehydes or ketones: Drag each item to the appropriate bin. View Available Hint(s) Reset Help Aldehyde Ketone Submit As text, an aldehyde group is represented as -CHO; a ketone is represented as -C(O)- or -CO-. In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp 2 hybridization. Two of the sp 2 orbitals on the carbon atom in the carbonyl group are used to form σ bonds to the other carbon or hydrogen atoms in a
.jpg)
Source Image: bartleby.com
Download Image
Classify The Following Line-Bond Formulas As Ketones Or Aldehydes
As text, an aldehyde group is represented as -CHO; a ketone is represented as -C(O)- or -CO-. In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp 2 hybridization. Two of the sp 2 orbitals on the carbon atom in the carbonyl group are used to form σ bonds to the other carbon or hydrogen atoms in a Copy Classily the following line-bond formulas as ketones or aldehydes. (Remember that carbon must have 4 bonds. If a line-angle t three bonds, the missing fourth bond is understood to go to a hydrogen atom) Drag each item to the appropriate bin. form ula shows a carbon atom with only View Available Hint (s) Aldenyde НН 335 PM 1/23/2018
Answered: Part A Classify the following line-bond… | bartleby
Campus Bookshelves Pasadena City College CHEM 001A: General Chemistry and Chemical Analysis 22: Organic Chemistry 22.10: Aldehydes and Ketones Expand/collapse global location 22.10: Aldehydes and Ketones Page ID Table of contents Uses of Aldehydes and Ketones: Compounds, Different Uses, Videos, Q&A

Source Image: toppr.com
Download Image
Ketone | Definition, Structure & Examples – Video & Lesson Transcript | Study.com Campus Bookshelves Pasadena City College CHEM 001A: General Chemistry and Chemical Analysis 22: Organic Chemistry 22.10: Aldehydes and Ketones Expand/collapse global location 22.10: Aldehydes and Ketones Page ID Table of contents

Source Image: study.com
Download Image
Aldehydes, Ketones and Carboxylic Acids – Practically Study Material Step 1 The aldehydes and ketones are the two types of organic functional groups. View the full answer Step 2 Final answer Previous question Next question Transcribed image text: B. Classify the following line-bond formulas as ketones or aldehydes Drag each item to the appropriate bin. Aldehyde Ketone mViewtassignmentPio
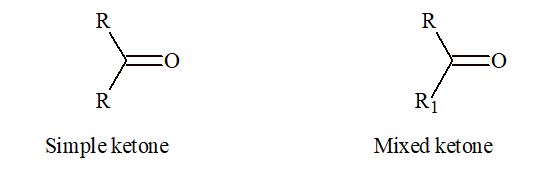
Source Image: practically.com
Download Image
SOLVED: Classify the following line-bond formulas as aldehydes or ketones: Drag each item to the appropriate bin. View Available Hint(s) Reset Help Aldehyde Ketone Submit 1: Aldehydes and Ketones 1.2: Aldehydes and Ketones– Structure and Names Expand/collapse global location 1.2: Aldehydes and Ketones– Structure and Names Page ID

Source Image: numerade.com
Download Image
a. Rewrite the following structual formula in bond line notation. (i Oct 27, 2022Tools expand_more Help expand_more Get Help Feedback Readability x selected template will load here Error This action is not available. chrome_reader_mode Enter Reader Mode Search Expand/collapse global hierarchy Home Bookshelves Introductory, Conceptual, and GOB Chemistry Basics of General, Organic, and Biological Chemistry (Ball et al.)

Source Image: doubtnut.com
Download Image
Aldehydes _ Ketones.doc As text, an aldehyde group is represented as -CHO; a ketone is represented as -C(O)- or -CO-. In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp 2 hybridization. Two of the sp 2 orbitals on the carbon atom in the carbonyl group are used to form σ bonds to the other carbon or hydrogen atoms in a

Source Image: slideshare.net
Download Image
Ketone | Definition, Structure & Examples – Video & Lesson Transcript | Study.com Copy Classily the following line-bond formulas as ketones or aldehydes. (Remember that carbon must have 4 bonds. If a line-angle t three bonds, the missing fourth bond is understood to go to a hydrogen atom) Drag each item to the appropriate bin. form ula shows a carbon atom with only View Available Hint (s) Aldenyde НН 335 PM 1/23/2018

Source Image: study.com
Download Image
Ketone | Definition, Structure & Examples – Video & Lesson Transcript | Study.com
Ketone | Definition, Structure & Examples – Video & Lesson Transcript | Study.com Jul 26, 2022search Search build_circle Toolbar fact_check Homework cancel Exit Reader Mode school Campus Bookshelves menu_book Bookshelves perm_media Learning Objects login Login how_to_reg Request Instructor Account hub Instructor Commons Search Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF) Resources expand_more
SOLVED: Classify the following line-bond formulas as aldehydes or ketones: Drag each item to the appropriate bin. View Available Hint(s) Reset Help Aldehyde Ketone Submit Aldehydes _ Ketones.doc Oct 27, 2022Tools expand_more Help expand_more Get Help Feedback Readability x selected template will load here Error This action is not available. chrome_reader_mode Enter Reader Mode Search Expand/collapse global hierarchy Home Bookshelves Introductory, Conceptual, and GOB Chemistry Basics of General, Organic, and Biological Chemistry (Ball et al.)