Step 1: First, convert the mass of solute to moles using the molar mass of HCl (36.5 g/mol): 22.4gHCl × 1molHCl 36.5gHCl = 0.614mol HCl. Step 2: Now we can use the definition of molarity to determine a concentration: M = 0.614molHCl 1.56Lsolution = 0.394MHCl.
CHEM 121 Lecture 15: The Mole Concept and Atoms- Chapter 4 Chemistry Notes – OneClass
So one mole Hcls equal to 36.5 g Hcl and one molecule of hcl Equal to 36 .5 Atomic Mass Units. And one atomic mass unit Is equal to 1.6605 times 10 to the -24g. That using these facts, Let’s calculate average natural number. So start with 36.5 g Hcl, one mole of hcl hands. This would be 36.5 atomic mass units. 21 molecule Hcl and one Atomic

Source Image: pt.slideshare.net
Download Image
For example, what is the molar concentration of a solution of 22.4 g of HCl dissolved in 1.56 L? Step 1: First, convert the mass of solute to moles using the molar mass of HCl (36.5 g/mol): 22.4gHCl × 1molHCl 36.5gHCl = 0.614mol HCl. Step 2: Now we can use the definition of molarity to determine a concentration:
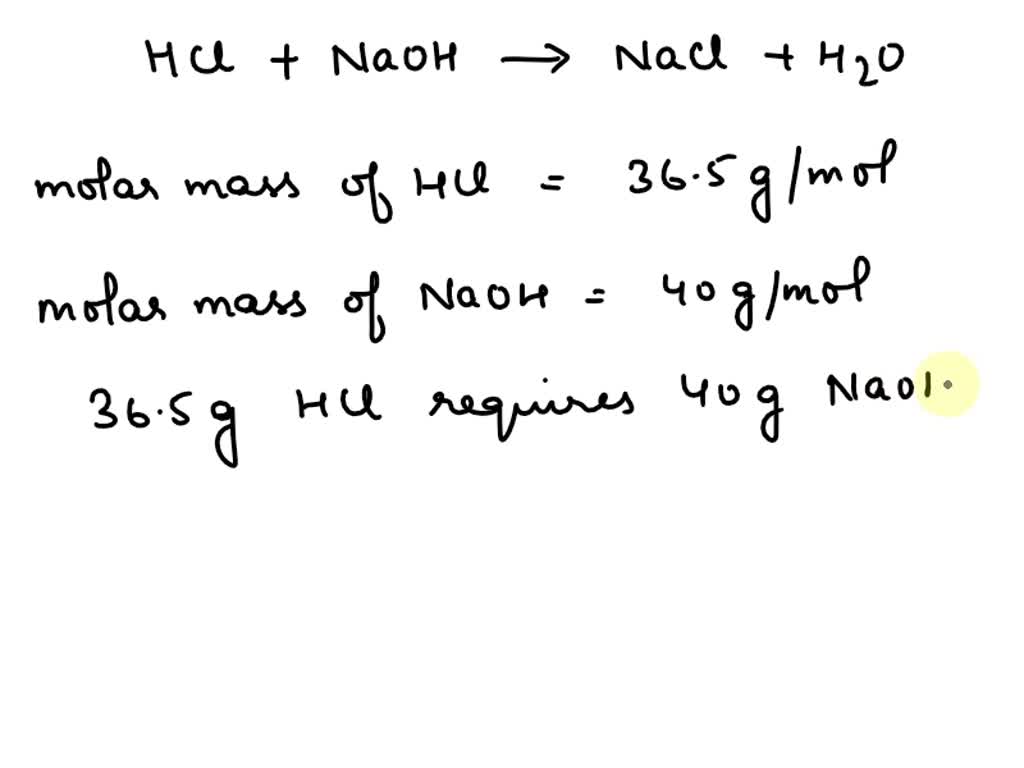
Source Image: numerade.com
Download Image
So I have made some HCl with sodium bisulfate and sodium chloride and a bit of water, in the end the water was 5ml and the HCl was about 45ml. Now the Chemistry 1 Answer anor277 Mar 14, 2018 [H Cl] = 11.7 ⋅ mol ⋅ L−1 Explanation: For molarity, we want the quotient…. molarity ≡ moles of solute (mol) volume of solution (L) …and thus we gets an answer with the required units of mol ⋅ L−1 …the volume of the solution is IRRELEVANT…. We work from a 1 ⋅ mL volume of solution

Source Image: chemistrybytes.com
Download Image
The Mole Of Hcl Is 36.5g How
Chemistry 1 Answer anor277 Mar 14, 2018 [H Cl] = 11.7 ⋅ mol ⋅ L−1 Explanation: For molarity, we want the quotient…. molarity ≡ moles of solute (mol) volume of solution (L) …and thus we gets an answer with the required units of mol ⋅ L−1 …the volume of the solution is IRRELEVANT…. We work from a 1 ⋅ mL volume of solution Science Chemistry Avogadro constant The molar mass of HCl is 36.5 g/mol, and the average mass per HCl molecule is 36.5 amu. Use the… Question: The molar mass of H C l is 36.5
Standard Temperature and Pressure | ChemistryBytes.com
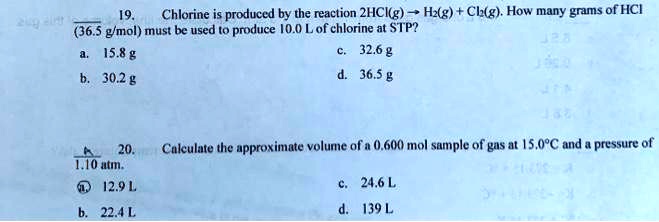
More information from the unit converter. How many grams HCl in 1 mol? The answer is 36.46094. We assume you are converting between grams HCl and mole.You can view more details on each measurement unit: molecular weight of HCl or mol This compound is also known as Hydrochloric Acid.The SI base unit for amount of substance is the mole. 1 grams HCl is equal to 0.027426610504282 mole. SOLVED: Chlorine is produced by the reaction 2HCl(g) → H2(g) + Cl2(g). How many grams of HCl (36.5 g/mol) must be used to produce 10.0 L of chlorine at STP? a) 15.8
Source Image: numerade.com
Download Image
find the number of molecules in 73 gm of HCl – Brainly.in More information from the unit converter. How many grams HCl in 1 mol? The answer is 36.46094. We assume you are converting between grams HCl and mole.You can view more details on each measurement unit: molecular weight of HCl or mol This compound is also known as Hydrochloric Acid.The SI base unit for amount of substance is the mole. 1 grams HCl is equal to 0.027426610504282 mole.

Source Image: brainly.in
Download Image
CHEM 121 Lecture 15: The Mole Concept and Atoms- Chapter 4 Chemistry Notes – OneClass Step 1: First, convert the mass of solute to moles using the molar mass of HCl (36.5 g/mol): 22.4gHCl × 1molHCl 36.5gHCl = 0.614mol HCl. Step 2: Now we can use the definition of molarity to determine a concentration: M = 0.614molHCl 1.56Lsolution = 0.394MHCl.

Source Image: oneclass.com
Download Image
So I have made some HCl with sodium bisulfate and sodium chloride and a bit of water, in the end the water was 5ml and the HCl was about 45ml. Now the For example, what is the molar concentration of a solution of 22.4 g of HCl dissolved in 1.56 L? Step 1: First, convert the mass of solute to moles using the molar mass of HCl (36.5 g/mol): 22.4gHCl × 1molHCl 36.5gHCl = 0.614mol HCl. Step 2: Now we can use the definition of molarity to determine a concentration:
Source Image: quora.com
Download Image
The value of heat generated when 36.5 g HCl and 40 g of NaOH react during neutralization (a) 76.5 kcal (b) 13.7 kcal (c) More than 13.7 kcal (d) 108 kcal di hatic eynansion it gets cooled Tyler DeWitt Do not sell my personal information The molar mass of HCl is 36.5 g/mol, and the average mass per HCl molecule is 36.5 u. Use the fact that 1 u = 1.6605 * 102 24g to calculate Avogadro’s number.

Source Image: toppr.com
Download Image
Concentration of Solutions: Definitions, Formulas, Solved Problems | Read Chemistry Chemistry 1 Answer anor277 Mar 14, 2018 [H Cl] = 11.7 ⋅ mol ⋅ L−1 Explanation: For molarity, we want the quotient…. molarity ≡ moles of solute (mol) volume of solution (L) …and thus we gets an answer with the required units of mol ⋅ L−1 …the volume of the solution is IRRELEVANT…. We work from a 1 ⋅ mL volume of solution

Source Image: readchemistry.com
Download Image
SOLVED: Describe the preparation of 100 ml of 6 M HCl from a concentrated solution that has a specific gravity of 1.18 and is 37 % (w/w) HCl (36.5 g/ mol). Science Chemistry Avogadro constant The molar mass of HCl is 36.5 g/mol, and the average mass per HCl molecule is 36.5 amu. Use the… Question: The molar mass of H C l is 36.5
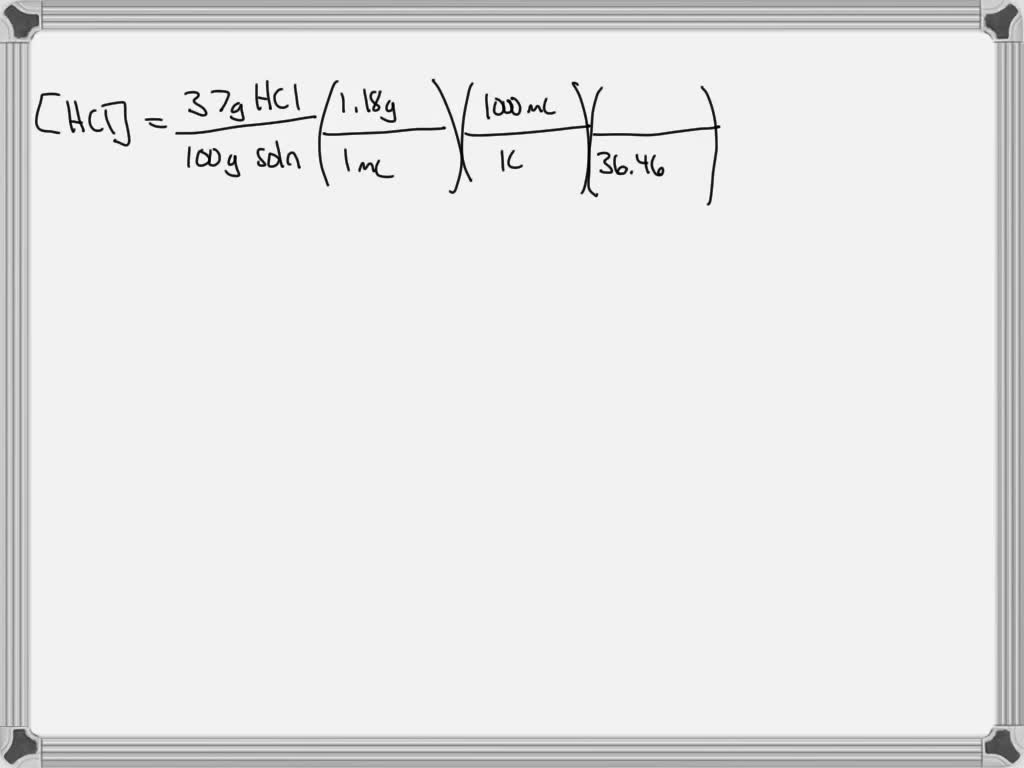
Source Image: numerade.com
Download Image
find the number of molecules in 73 gm of HCl – Brainly.in
SOLVED: Describe the preparation of 100 ml of 6 M HCl from a concentrated solution that has a specific gravity of 1.18 and is 37 % (w/w) HCl (36.5 g/ mol). So one mole Hcls equal to 36.5 g Hcl and one molecule of hcl Equal to 36 .5 Atomic Mass Units. And one atomic mass unit Is equal to 1.6605 times 10 to the -24g. That using these facts, Let’s calculate average natural number. So start with 36.5 g Hcl, one mole of hcl hands. This would be 36.5 atomic mass units. 21 molecule Hcl and one Atomic
So I have made some HCl with sodium bisulfate and sodium chloride and a bit of water, in the end the water was 5ml and the HCl was about 45ml. Now the Concentration of Solutions: Definitions, Formulas, Solved Problems | Read Chemistry Tyler DeWitt Do not sell my personal information The molar mass of HCl is 36.5 g/mol, and the average mass per HCl molecule is 36.5 u. Use the fact that 1 u = 1.6605 * 102 24g to calculate Avogadro’s number.